Description
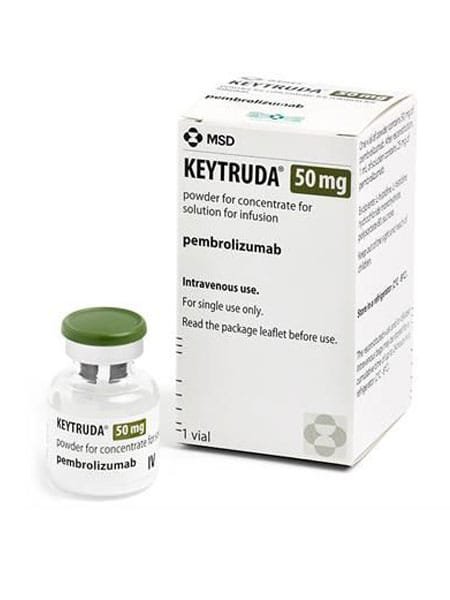
Pembrolizumab, marketed under the brand name KEYTRUDA, It is an prescription medication that has been approved pembrolizumab (Keytruda) for a number of uses at various dates, including:
October 24, 2016
Approved for treating patients with metastatic non-small cell lung cancer (mNSCLC) whose tumors express PD-L1
July 26, 2021
Approved for treating high-risk, early-stage triple-negative breast cancer (TNBC)
March 29, 2023
Approved for treating adults and children with unresectable or metastatic microsatellite instability–high (MSI-H) solid tumors, and mismatch repair–deficient (dMMR) solid tumors
November 16, 2023
Approved for treating adults with locally advanced unresectable or metastatic HER2-negative gastric or gastroesophageal junction (GEJ) adenocarcinoma
June 17, 2024
Approved for treating adults with primary advanced or recurrent endometrial carcinoma.
What is Keytruda (Pembrolizumab) ?
KEYTRUDA (pembrolizumab) injection, for intravenous use. Initial U.S. Approval: 2014.
Keytruda is classified as a monoclonal antibody. Monoclonal antibodies are a relatively new type of “targeted” cancer therapy. Antibodies are an integral part of the body’s immune system. Normally, the body creates antibodies in response to an antigen (such as a protein in a germ) that has entered the body. The antibodies attach to the antigen in order to mark it for destruction by the immune system.
Pembrolizumab is a cancer medicine that interferes with the growth and spread of cancer cells in the body. It is used to treat advanced skin cancer (melanoma) that has spread to other parts of the body. It is also used to treat a certain type of non-small cell lung cancer, if your tumor has a specific genetic marker for which your doctor will test. Keytruda Pembrolizumab is also used to treat head and neck cancer that has spread to other parts of the body, or has come back after prior treatment. Pembrolizumab is also used to treat Hodgkin lymphoma in adults and children.
How to order approved “Keytruda (Pembrolizumab) for Injection” Medicine?
KEYTRUDA (Pembrolizumab) Injection is a prescription medicine, it can be accessed through the Named Patient Program (NPP) for treatment in the following city of India – Bengaluru, Delhi, Chennai, Hyderabad, Mumbai, Pune, Kolkata, Ahmedabad, Gurgaon. To inquire about the cost price of purchasing KEYTRUDA and arranging delivery to your location, please get in touch with Mr. Rakesh at +91 9910645395 or send an enquiry to info@cancermedicinesnetwork. We assure you of the quality of the product and guarantee delivery in accordance with your specific requirements.